
VIASURE Real Time PCR Detection Kits
Meningitis Panel 01
Description
VIASURE Viral Meningitis Panel Real Time PCR Detection Kit is a non-automatised RT-qPCR reactions designed for the simultaneous qualitative detection and differentiation of DNA/RNA from Human Herpesvirus 6 (HHV-6), Human Herpesvirus 7 (HHV-7), Human Herpesvirus 8 (HHV-8), Herpes simplex virus type 1 (HSV- 1), Herpes simplex virus type 2 (HSV-2), Varicella zoster virus (VZV), Mumps virus (MuV), Enterovirus (HEV), Parechovirus (HPeV), Adenovirus (ADV), Cytomegalovirus (CMV), Epstein-Barr virus (EBV) and Parvovirus B19 (PB19), viruses that cause viral meningitis or signs and symptoms showing neurological involvement, in EDTA-plasma and cerebrospinal fluid samples (CSF) from individuals suspected of neurological symptoms by their healthcare provider (HCP).
This test panel is intended to be used as an aid in the diagnosis of these viruses’ infection in combination with clinical and epidemiological risk factors. DNA and RNA are extracted from clinical samples, and then the RNA is retrotranscribed into complementary DNA (cDNA), using a reverse transcriptase (RT) enzyme. The DNA and cDNA are amplified using real time PCR and detected using fluorescent reporter dye probes specific for each virus.
The product is intended for use by qualified and trained clinical laboratory personnel specifically instructed and trained in the techniques of real-time PCR and in vitro diagnostic procedures (including training on the Real Time PCR instrument (thermocycler) and nucleic acid extraction system).
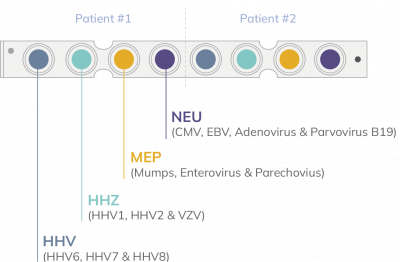
Information about targets, channels and genes included in the design of the VIASURE Viral Meningitis Panel Real Time PCR Detection Kit is included in Annex 1 for open format with internal control products and Annex 2 for open format with extraction control products.

